- Feb. 11, 2019
- BugSpeaks
- Gestational and Neonatal
The gut microbiome alterations is related to autism spectrum disorders
Autism spectrum disorder (ASD) is a hereditary linked disorder of brain function with symptoms, such as restricted forms of behaviors and activities, or persistent discrepancies in societal interaction and communication. ASD has a significant impact on the growth and development of children and on the general public. The assessed incidence of ASD in 2012 was about 14.6 per 1,000 children (aged 8 years), and its occurrence was relatively higher in boys (23.6 per 1,000) when compared to girls (5.3 per 1,000). As per the estimate, in the United States alone, the cost of caring for a child with ASD was about $1.4 million, while in the United Kingdom, it was reported to be £0.92 million (Li et al. 2017). The main costs associated with the care of children with ASD are special education services and a loss of parental productivity. Thus, the financial effects of ASD have driven scientists to hunt for effective medications (Buescher et al. 2014; Li et al. 2017).
ASD is described by numerous clinical endophenotypes, and is hypothetically connected with certain comorbidities. As per the current recommendations, gastrointestinal symptoms are the reasons for the comorbidity in patients with ASD, however underlying mechanisms are unfamiliar. Several investigations have revealed that patients with ASD have alterations in the fecal flora composition, and the gut microbiome metabolic products. The dietary constituents along with metabolic activities of the microbiome are now assumed to be linked to alterations in behavior and cognition, mainly in patients having neurodegenerative diseases. The gut microbiome have a significant role in the development of the brain and controls the behavior via the neuroimmune, neuroendocrine, and autonomic nervous systems. There is a bidirectional communication between the central nervous system and the gastrointestinal tract (brain-gut axis) and the gut microbiome functions. As stated by most recent studies, collective pathogenetic elements and pathophysiological mechanisms probably associating to gastrointestinal disturbances and ASD include intestinal inflammation with or without autoimmunity, gluten-related disorders (celiac disease, wheat allergy, non-celiac gluten sensitivity), immunoglobulin E-mediated and/or cell-mediated gastrointestinal food allergies, abdominal pain, dysautonomia connected with gastroesophageal reflux and gastrointestinal dysmotility. In addition, dysregulation of the gut microbiome has the capability to upset intestinal permeability, motility and sensitivity, and mucosal immune function.
The human gut contains about 1000 grams of bacteria, and approximately about 9.9 million numbers of bacterial genes exist in the gut i.e., the ratio of microbiome DNA and host DNA is 10:1. Gastrointestinal indications are noticeable in ASD patients. Researchers have observed more gastrointestinal syndromes, including diarrhea and constipation in children having ASD compared with their unaffected siblings. ASD patients with gastrointestinal syndromes display substantial interactive or behavioral manifestations, including self-injury, aggression and anxiety. Many studies have demonstrated that the gut microbiota composition and function is directly or indirectly linked with ASD signs, partly by prompting the immune system and metabolic rate. Patients with ASD are observed to have a higher percentage of abnormal intestinal permeability leading to greater antigenic load from the gastrointestinal tract. ASD-associated cytokines, such as interferon-γ (IFN-γ), interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) in addition to lymphocytes present in the circulation cross the blood-brain barrier (BBB). Successively, IL-6, IL-1β and TNF-α bind to brain endothelial cells and encourage immune responses in the brain. Variations in the gut microbiota composition and their metabolic products secretion are generally noticed in ASD patients and in animal models of ASD. In a mouse model study featuring ASD, researchers have found that gastrointestinal barrier defects caused microbiota alterations. They observed more abundant bacteria, belonging to Prevotellaceae, Porphyromonadaceae, unclassified Bacteroidales and Lachnospiraceae in offspring of mothers with maternal immune activation, while in control offspring, Erysipelotrichaceae, Ruminococcaceae and Alcaligenaceae were found to occur more abundantly. The gut microbiota diversity in children with ASD is observed to be very less as compared to that of children without ASD. In ASD patients, higher levels of Desulfovibrio, Lactobacillus, Bacteroidetes, Clostridium, Caloramator and Sarcina, and lower levels of Bifidobacterium and Firmicutes are observed. Further, children with ASD and gastrointestinal syndromes possess inferior loads of unclassified Veillonellaceae and the genera, Coprococcus, and Prevotella than that found in gastrointestinal syndrome-free neurotypical children. Clostridium histolyticum, found in the fecal samples of children with ASD was at a higher level when compared with healthy children without ASD. Moreover, altered levels of Prevotella, Bifidobacterium, and Sutterella are found in children with ASD. Researchers also have observed that Candida species (Candida krusei and C. glabrata) were 2-times higher in individuals affected with autism than in normal individuals. Also, it is observed that Candida species can release ammonia and toxins that can encourage autistic behaviors.
The gut–brain axis is viewed as a way of interaction between the gut microbiome and the brain, and it is a two-way communication structure. The outcome of several studies suggests that the gut-brain axis contributes to the origin and development of ASD. In a comprehensive review by Li et al. (2017) stated that the gut flora affects functions of the brain via the neuroendocrine, neuroimmune and autonomic nervous systems and through toxin production by microbiota (Figure 1). The presence of millions of neurons in the mucosa of the gastrointestinal tract constitutes the enteric nervous system and modulate various gastrointestinal functions. Hence, the gut is recognized as ‘the second brain’. ?The potential interactions between the gut microbiota and the pathogenesis of ASD is outlined in Figure 1.
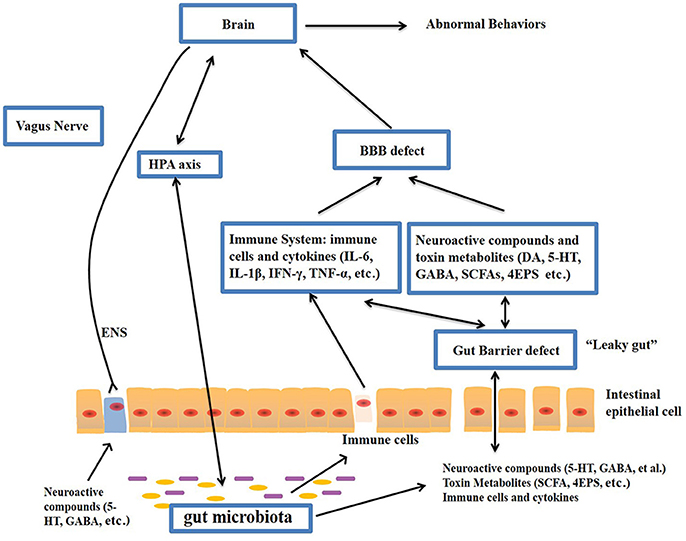
Figure-1: Potential relationships between the microbiota and ASD (the gut-brain axis). The production of metabolites, such as SCFAs and toxin metabolites, by certain microbiota (e.g., Lactobacillus) can cross the “leaky gut” to affect brain function. Some microbiota can produce neuroactive compounds (e.g., 5-HT and GABA) that cross the “leaky gut” and influence brain function and induce abnormal behaviors. These neuroactive compounds can directly influence the HPA axis and increase circulating levels of cortisol. Metabolites, certain microbiota and neuroactive compounds can activate enteric neurons and affect brain function through the vagus nerve. Some microbiota and metabolites can activate gut immune cells, which can release cytokines into circulation. 4-EPS, 4-ethylphenyl sulfate; 5-HT, serotonin; HPA, hypothalamic–pituitary–adrenal; SCFAs, short-chain fatty acids; BBB, blood-brain barrier; 5-HT, 5-hydroxytryptamine; ENS, enteric nervous system; GABA, γ-aminobutyric acid; DA, dopamine. (Adapted from Li et al. (2017); doi: 10.3389/fncel.2017.00120)
The gut microbiota secretes certain metabolites, such as phenolics, short-chain fatty acids (acetic acid, proprionic acid, isobutyric acid, butyrate, valeric acid and isovaleric acid), and free amino acids that affect ASD-like behaviors via the vagal pathways mediating the microbiome-brain-gut axis communication. Likewise, the gut microbiota releases neuroactive compounds (dopamine, 5-HT, γ-aminobutyric acid, and histamine), which trigger or impede central neurons via the vagus nerve.
Currently, there are no available effective therapies for ASD. Parents often take their children to obtain mediations that are personalized to their specific needs. Recent evidences have indicated that the gut microbiota modulation can be a potential therapy in children having ASD. In this regard, prebiotics, probiotics, fecal microbiota transplantation and a suitable personalized diet have gained substantial attention in recent days. Moreover, microbiome-facilitated therapies are relatively safe and effective. Recent clinical studies have reported that ASD patient’s symptoms can be improved by treatments that normalize the gut microbiota. Nevertheless, more research studies involving more participants are required to know about the treatments that supports the gut microbiome in overcoming ASD symptoms.
Keywords:
The gut microbiota, microbiome, Autism spectrum disorder, Brain-gut axis, Probiotics, Fecal microbiota transplantation, Microbiome-mediated therapies
References:
- Li Q, Han Y, Dy AB, Hagerman RJ. The gut microbiota and autism spectrum disorders. Frontiers in cellular neuroscience. 2017 Apr 28;11:120.
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior. 2015 Jan 1;138:179-87.
- Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA pediatrics. 2014 Aug 1;168(8):721-8.