- Jan. 23, 2020
- BugSpeaks
- Microbiome and Disease
Crohn's disease: No reason to be Hopeless
Our gut harbours diverse flora working like an organ system made up of bacteria, viruses, fungi, and several other microbes. They perform a variety of functions, such as training our immune system and suppressing harmful microorganisms. But when the balance in the delicate microbial world tips off, dysbiosis occurs, paving the way to an array of health conditions. Crohn’s disease is one such condition wherein the impairment of gut microbiota causes a whole lot of pain to an individual. Down goes the number of healthy bacteria, like, Firmicutes and Bacteroides, and pathogenic bacteria, Gammaproteobacteria, Bacteroidales, Erysipelotrichales overpopulate the gut.
Crohn’s disease includes long term inflammation in bowel with intermittent aggravation and remission. This illness affects different segments of the digestive tract in different people depending on the severity and activity of the disease. Patients suffer from pain in the abdomen, severe diarrhoea, mouth sores, tiredness, malnourishment, and excessive weight loss. Crohn’s disease may either be a fault of inherited genes or the immune system. The exact causation remains unknown. Diet rich in spices, processed food, and stressful lifestyle often worsens the situation. Quite often, a deeper layer of the gut tissue gets involved, which can pose a threat to life.
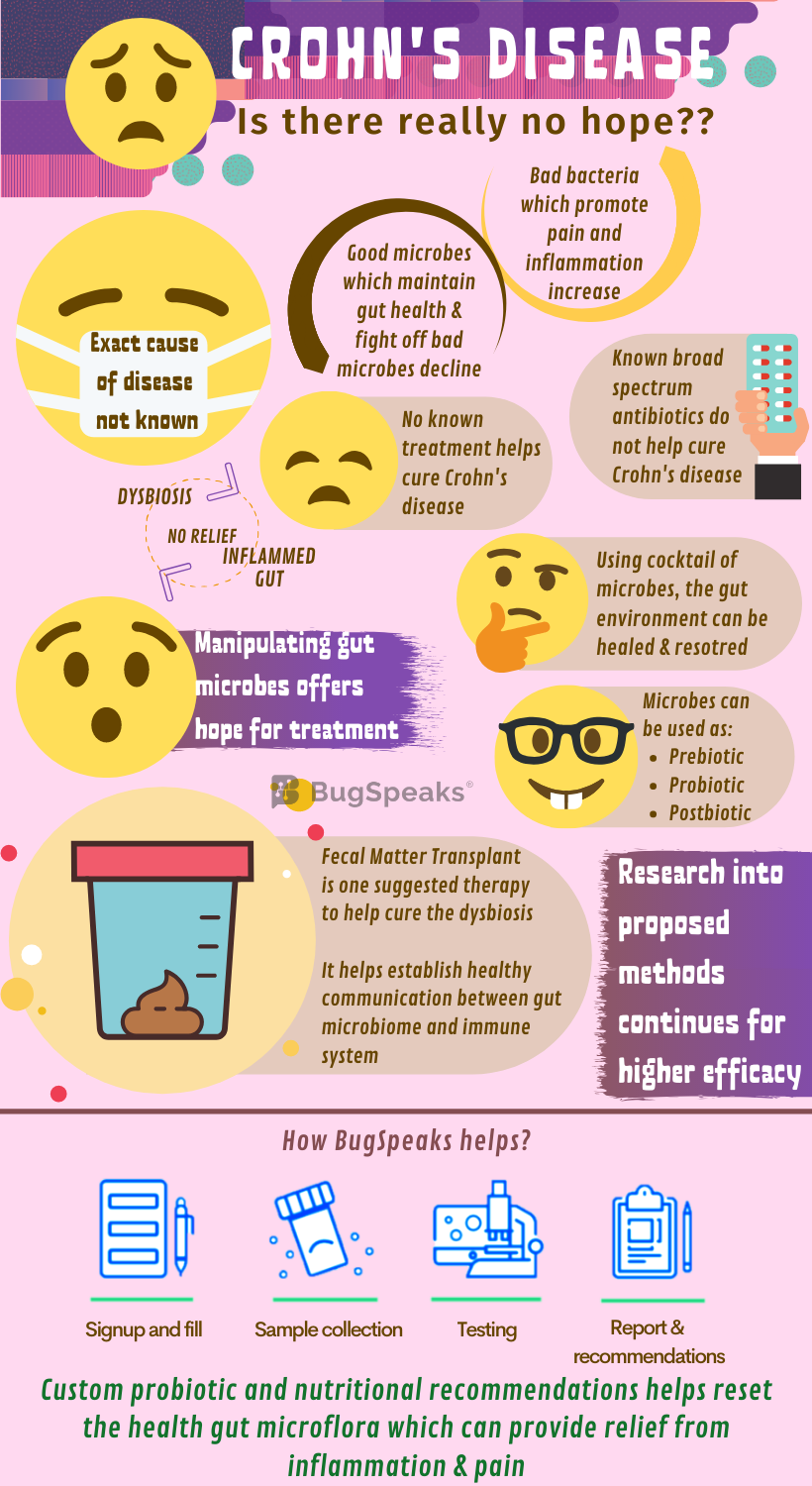
The relation between Crohn's and gut microbiome
Some friendly members of gut flora like Faecalibacterium, Phascolarctobacterium, and Roseburia ferment dietary fibre to produce short-chain fatty acids (SCFAs). SCFAs like butyrate, propionate, and acetate provide energy and immunity to the colon epithelium. Sadly, SCFA producing bacteria decline in the diseased gut. Moreover, Lactobacillus and Bifidobacterium populations, which promote protection against gut mucosal inflammation, also decline. Like bacteria, numbers of beneficial fungi also fluctuate.
For example, Dioszegia and Candida glabrata assume predominance, whereas Trichosporon and Leptosphaeria become a minority. Once imbalanced, the disturbed microbial diversity further alters the gut environment even during the remission period. For example, the neutral and acidic mucin - a key component of mucus in the gut membrane - secretion remains high during the remission period. It is due to a reduction in bacteria of genera Oscollospira and Akkermansia, sulphate-reducing bacteria and Saccharomyces cerevisiae and does not allow relief from inflammation.
Pain with no treatment?
Treating Crohn's diseases is as easy as soft-landing a rover on the moon's lunar surface. No one has done it, yet the efforts and research continue. Antibiotics, your mate in fighting most of the diseases, proves inadequate in Crohn’s disease. The broad-spectrum nontargeted microbiome-destroying approaches do not serve the purpose. Increasing knowledge into the promising role of gut microbiota in the plethora of conditions now poses a hopeful avenue for Crohn's patients. The intriguing role of gut microbiota in host immune response ways may hold the key to their function in controlling gut immunity and thus health restoration.
Manipulation of gut microbes: A hope for pain
Aimed at manipulation of the gut microbial population efforts to harness prebiotics, probiotics, and postbiotics to harmonize the gut habitat in Crohn’s disease continues. Prebiotics promote the growth and colonization of gut-friendly organisms. fibre remains as one of the oldest and most frequently used prebiotics. Some beneficial organisms have the unique ability to ferment indigestible fibre to produce SCFA critical to a healthy colon. So, logically, a fibre-rich diet would lower the risk of Crohn’s disease and would have been a line of management in affected people. However, enough evidence lacks this support. Further studies for a better understanding and utilization of fibres in Crohn's disease management remain warranted.
Knowing the association of Crohn’s disease with dysbiosis, the administration of probiotic strains to help stabilize the gut microbiome seems rational. It may also help redesign the gut habitat in a more advantageous way. This holds the reasonable potential to improve the disease condition. Thus, therapies to restructure microbial communities could be used for improved management of Crohn’s disease. However, more data can help establish the role of probiotic administration as a therapy. Also, the negligible clinical achievement with probiotics shows that single or multiple non-target probiotic strain administration may not be helpful. To sustain this treatment, the cocktail of targeted micro-organisms should be used.
One man's waste another man's treasure
Another strategy includes the use of postbiotics, the bioactive molecules produced by a microbial community, which maintain the health of the gastrointestinal tract. With the latest cutting-edge technology, identification of vital metabolic pathways and bioactive postbiotics is possible. Administration of postbiotics to patients with Crohn’s disease may help re-establish the full functioning gut microbial community. Faecal microbiota transplantation (FMT) is another possible choice of therapy in Crohn’s disease and other diseases with altered microbial compositions.
Crohn’s disease has complicated pathogenesis involving host genetics and dysregulation of the host-microbiome crosstalk. The aim of FMT in Crohn’s disease is to cure dysbiosis and re-establish healthy communication between the host immune system and the microbiota. However, due to the complication of the underlying genetic predisposition of the host, success remains limited in this case. However, the long-term consequence of FMT as a strategy to treat Crohn’s disease is still not clear. In few years down the lane, as a better approach, FMT will inevitably be replaced with a well-defined blend of micro-organisms.
Gut microbiome testing: A personal plan to eliminate Chron's disease
The perturbation or the disturbance of gut flora in Crohn’s disease presents complexity and the mechanisms not yet fully understood. Moreover, it has not been possible to dissect out the dysbiotic changes that, as a consequence, may lead to mucosal inflammation. Severe and chronic inflammation of Crohn’s disease alters intestinal flora. This modification, in turn, leads to atypical host-microbiome interactions, ultimately exacerbating the primary disease. Possibly, through regulation of inflammation or control over the host-microbiome interaction may help disrupt the vicious circle of inflammation and dysbiosis.
As Crohn’s disease and gut microbiome are entangled, so, to get resolved, it is a prerequisite to taking a ‘Gut Microbiome Test.’ With the help of the latest ‘DNA sequencing technology,’ the exclusive array of micro-organisms in patient stool samples are identified. Then the diet/ therapeutic plan is processed based on the unique microbial population harbouring in the gut. The complete cure of Crohn's disease is so far, not known. But there is hope. Like all chronic illnesses, the only way is to reduce the signs and symptoms significantly. Even long-term remission may be attained through the help of one single therapy or multiple therapies simultaneously or separately. With proper treatment, many people with Crohn's disease can function well and lead an active life.