- Feb. 6, 2020
- BugSpeaks
- Microbiome and Disease
Irritable gut - Irritable you
Like us, our bowel can get irritated too!
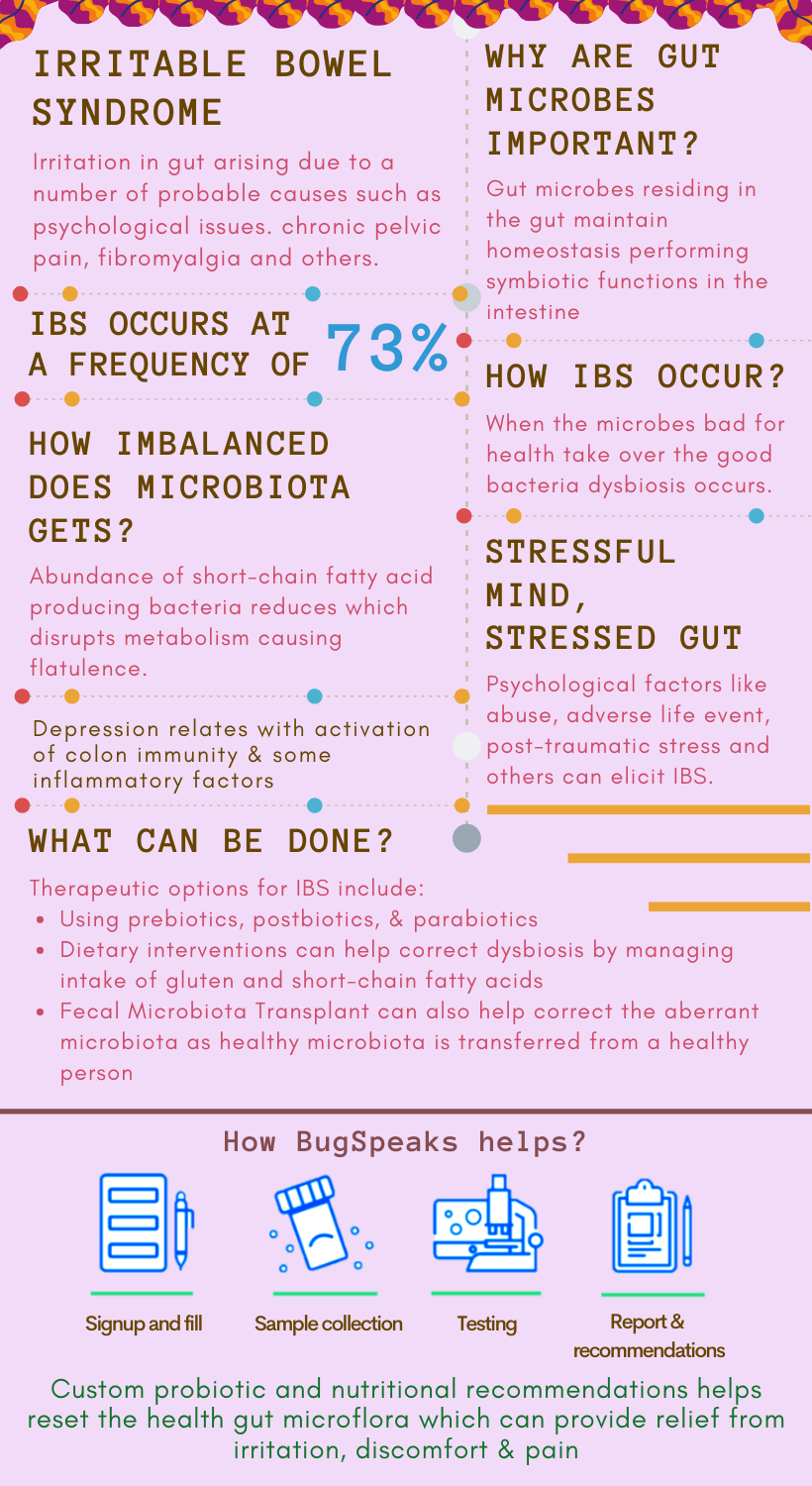
In one-word, irritation in our gut may indicate ‘Irritable bowel syndrome’ (IBS). Often associated with chronic, recurrent abdominal discomfort and pain, IBS gets worse after meals and resolves upon defecation. Symptoms include changes in bowel habits, diarrhoea, constipation, or alternating constipation and digestive symptoms, such as dyspepsia, dysphagia, and nausea.
Sometimes, IBS finds a link with non-gastrointestinal disorders such as chronic pelvic pain, fibromyalgia, chronic fatigue syndrome, anxiety, and even depression. If unaddressed, these comorbidities may hamper your day-to-day life. A flare-up may last for several days, and then symptoms either improve or resolve completely. Don’t worry. Severe complications are rare in reality. Also, many such comorbidities often find an association with gut dysbiosis.
Why??
A complex assemblage of trillions of micro-organisms including fungi, eukaryotes, bacteria, viruses, and archaea live in our gut. These dynamic and vibrant microbial communities comprise of pro- and anti-inflammatory bacteria and maintain gastrointestinal homeostasis. This homeostasis enables microbes to colonize the intestine and perform symbiotic functions persistently. Thus, gut microbes are the critical players in IBS pathogenesis.
How??
Dysbiosis, typically associated with IBS, occurs at a frequency of 73%. Pre-dominant bacteria contributing to dysbiosis include Bacillus and Ruminococcus gnavus, Shigella or Escherichia, and Actinobacteria. Frequently, in IBS, commensals turn pathogenic through the gain of virulence. For example, Enterococcus faecalis turns to non-invasive E. coli, evading the immune system in disease pathogenesis. All this evidence indicates a strong relation between dysbiosis and the severity of IBS.
A little off-balance, a lot of imbalance
Certain gut bacteria ferment polysaccharides to produce SCFAs and gasses such as hydrogen (H2) and methane (CH4). Thus, they influence bowel movement and permeability of the intestinal barrier. However, a breach may occur in the intestinal wall due to the influx of inflammatory mediators or pathogens. Consequently, severe inflammation occurs, affecting the gut milieu with alteration of gut flora. As expected, a lower abundance of SCFA producing bacteria occurs in IBS, which also maintains lower counts of methanogens. Accordingly, microbial metabolism disrupts, resulting in a high production of hydrogen gas, causing flatulence in the abdomen.
Moreover, IBS subjects relative to healthy controls present overpopulation of Proteobacteria, Veillonella, Firmicutes, Lactobacillus, and Ruminococcus. They show a decreased quantity of Bifidobacterium, Faecalibacterium, Erysipelotrichaceae, and Methanogens. Moreover, the severity of IBS finds a positive correlation with a specific intestinal microbiota signature, characterized by low microbial richness, Methanobacteriales absence, and enriched Bacteroides.
Psychological Association
A stressful mind stresses your gut, so remains the case with IBS. Considered a stress-sensitive disorder, psychological factors like any abuse, adverse life event, and post-traumatic stress disorder can elicit IBS. How? These conditions are likely to be involved in gut-brain interaction and IBS pathogenesis. IBS and gut dysbiosis find a link with the psychological wellbeing of an individual. A prominent example relates to depression resulting from the activation of colon immunity and the release of certain inflammatory factors. Another common issue of high stress leads to altered metabolism of serotonin, a neurotransmitter that is responsible for both variations of gut motility and pathogenesis of IBS.
Genetic predisposition
Specific genetic aberrations like a mutation in the SC5NA gene responsible for immune regulation and epithelial barrier function relate to IBS development. Additionally, epigenetic factors also influence IBS occurrence.
Therapeutic Options
Probiotics are one of the essential therapeutic options. They play a role in modulating gut inflammation and produce antimicrobial peptides. These products help eliminate pathogenic bacteria and improve the mucosal barrier function easing out the symptoms of IBS. Consistent use of probiotics, the Biofidobacterium or Lactobacillus, or a blend of bacteria consisting of Bifidobacterium, Lactobacillus, and Streptococcus help improve the symptoms of IBS. However, due to a short lifespan, repeated doses of prebiotics are required. Therefore, prebiotics may serve as a better alternative treatment as they provide substrates that can help in the growth of specific bacteria and hence can alter the microbiota.
Prebiotics present multipronged benefits. For example, the prebiotic lactulose promotes gut bacteria, water retention in stool, exhibiting laxative effects. Other synthetic prebiotics includes various kinds of oligosaccharides like fructo-oligosaccharides, soybean oligosaccharides, galacto-oligosaccharides, isomalto-oligosaccharides, xylo-oligosaccharides, and others. Accordingly, cereals, fruits, and vegetables are rich in prebiotics. Commensal bacteria in the colon ferment prebiotics to produce SCFAs, which help regulate inflammatory responses, and influence gut homeostasis. Resultantly, dysbiosis gets corrected through the promotion of positive alterations in the gut microflora. A step ahead of probiotics and prebiotics, synbiotics, a combination of the two, show higher potency compared to either of the two.
A step ahead pro- and prebiotics
Another advancement over probiotics includes paraprobiotic and postbiotic. Paraprobiotics are non-viable or inactivated probiotics, while postbiotics are non-viable soluble factors secreted by live bacteria or released upon cell lysis. They benefit the host through their biological activity. The advantage of postbiotics and paraprobiotic lies in their safety profile and longer shelf-life while conferring health benefits comparable to those of probiotics. Moreover, some poorly/non-absorbed antibiotics with low toxicities, such as rifaximin and neomycin, also prove useful for the treatment of IBS. These antibiotics cause remarkable improvements in some instances of IBS known as small intestinal bacterial overgrowth.
Dietary Influence
Dietary interventions are helpful can help IBS patients as, in most cases, the consumption of certain foods causes aggravation of symptoms. In terms of dietary influences, the main culprits are gluten and short-chain carbohydrates that trigger harmful alterations. A diet that corrects the microbiota dysbiosis could thus help manage IBS symptoms. However, meagre consumption of short-chain carbohydrates may reduce the abundance of healthy gut commensal bacteria. Therefore, careful modification or restriction of dietary intake in IBS patients only proves helpful. But, to achieve an effective diet, an individual may need to go through a process of trial and error as IBS has no universal diet.
Faecal Microbiota Transplant (FMT)
Interestingly, some other person's excreta can also provide relief from IBS. Although it sounds a bit wicked, the poo treatment, known as Faecal Microbiota Transplant (FMT), can help. FMT involves applying a solution of faecal material from a healthy donor into the gut of a receiver, intending to restore the aberrant microbial composition. The technique aims to restore the dysbiosis in the gut.
Finally,
As science continues to disentangle the mysteries of the gut microbiome, the mechanisms to manipulate the gut microbiome for better health continue to widen. With the help of a ‘Gut Microbiome Test,’ it is very easy to obtain an individualized microbiome profile. Through DNA sequencing technologies, experts can now devise personalized tailor-made treatment diet plans, probiotic plans as per the need of the individual patients. There is no cure for IBS. However, as IBS is a chronic condition, so you need to manage it on a long-term basis. It can be kept under control by following the doctor’s advice religiously, maintaining diet, lifestyle, and stress level.